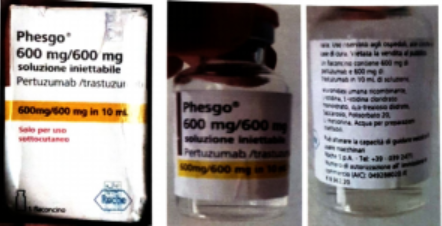
A warning has been issued by the National Agency for Food and Drug Administration and Control (NAFDAC) about a fake batch of Phesgo® 600mg/600mg/10ml injection, identified by batch number C5290S20.
The alleged counterfeit medication was brought in by a patient and reported by a physician at Lagos University Teaching Hospital (LUTH-NSIA). Examining the product revealed that it matched the C3809C51 counterfeit batch that had previously been found.
The Marketing Authorisation Holder, Roche, examined photos of the suspected product and verified that it was a fake, pointing out discrepancies such as a missing tamper-evidence feature, inappropriate language, a label that didn’t match the real product, and a batch number that didn’t exist.
Healthcare providers, retailers, importers, distributors, and carers are all urged by NAFDAC to use caution throughout the supply chain and refrain from importing, selling, distributing, or utilising counterfeit goods.
Every medical product should be purchased from approved or authorised vendors, and its physical state and validity should be thoroughly examined.
Consumers and healthcare professionals are encouraged to contact the closest NAFDAC office, call 0800-162-3322, or send an email if they suspect the sale of counterfeit or subpar medications or medical devices.
NAFDAC has instructed all zonal directors and state coordinators to carry out surveillance and take the fake goods out of distribution. The agency reassures the public that efforts are being made to detect and eradicate counterfeit goods.